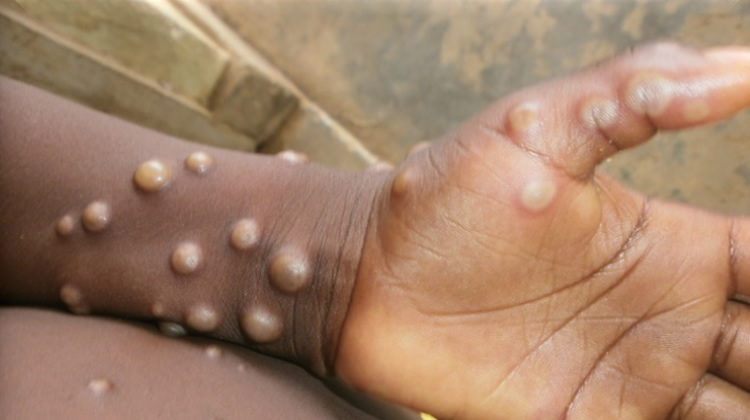
Gavi, the Vaccine Alliance, has announced plans to secure 500,000 doses of the Modified Vaccinia Ankara-Bavarian Nordic mpox vaccine for African countries affected by the monkeypox outbreak. This move is aimed at addressing the growing number of mpox cases in the region, with 26,544 reported cases, including 5,732 confirmed cases and 724 deaths, across 15 African Union member states as of week 35 in 2024.
The vaccine doses, scheduled for delivery in 2024, will be funded by Gavi’s First Response Fund, a new financial mechanism created in June 2024 to provide rapid funding for vaccine purchases during health emergencies. The MVA-BN vaccine has received prequalification from the World Health Organization and is the only non-replicating mpox vaccine approved in the U.S., Switzerland, and Singapore.
Gavi’s CEO, Dr. Sania Nishtar, emphasized the organization’s commitment to protecting those most at risk and building a global vaccine stockpile. Bavarian Nordic’s President & CEO, Paul Chaplin, expressed pleasure in signing the agreement, highlighting the significance of the doses secured through this agreement in increasing the availability of mpox vaccines for African countries.
In addition to activating the First Response Fund, Gavi moved quickly to trigger the mechanism following the declaration of mpox as a public health emergency. The organization unlocked emergency funding for affected countries to begin preparations for vaccine rollout, such as training healthcare workers and engaging communities. Gavi is also working with donors and partners to help facilitate dose donations.
This Advance Purchase Agreement follows the delivery of over a quarter of a million doses of Bavarian Nordic vaccines to the Democratic Republic of the Congo, donated by other nations and Bavarian Nordic. The doses will be allocated to those most in need according to WHO’s Access and Allocation Mechanism.