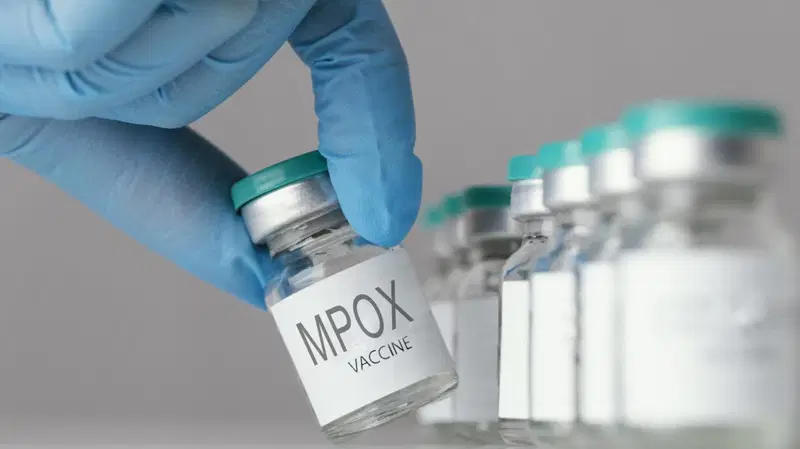
The World Health Organization (WHO) has prequalified the first mpox vaccine, MVA-BN, paving the way for accelerated access to the jab to combat the ongoing epidemic in Africa. This approval is expected to speed up procurement, donations, and rollout, ensuring equitable access to vaccines where they are needed most.
WHO’s prequalification listing evaluates the quality, safety, and efficacy of medical products, enabling the United Nations and other international agencies to procure them. Lower-income countries can also use this listing to fast-track procurement approvals.
The approval comes as the Democratic Republic of Congo, the epicenter of the epidemic, receives its first shipment of MVA-BN vaccines. The WHO has declared an international emergency over mpox, concerned by the surge in cases of the new Clade 1b strain in the DRC, which has spread to nearby countries, resulting in nearly 22,000 cases and 716 deaths since January.